Metallic bonding (IGCSE)
Supplement
• Describe metallic bonding as a lattice of positive ions in a ‘sea of electrons’ and use this to describe the electrical conductivity and malleability of metals.
Example of metallic sturctures in sodium and calcium.
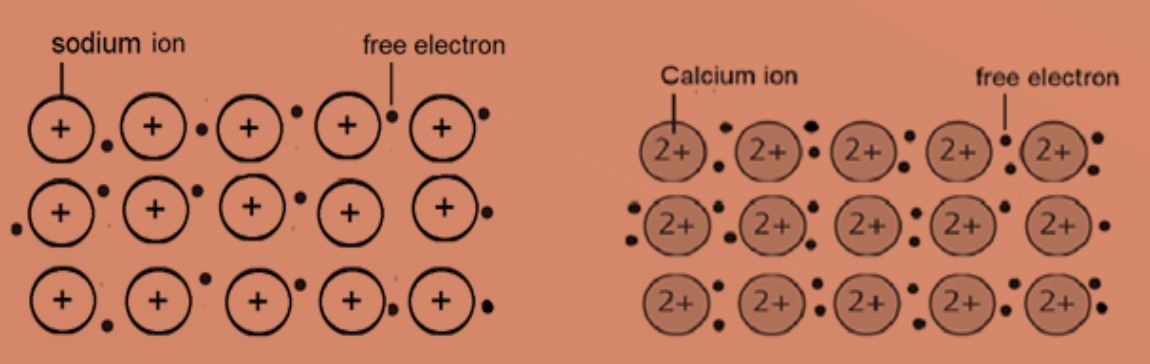
The atoms are packed closely in a regular lattice structure. The valence electrons of the atoms are free to move in the lattice structure resulting in a sea of delocalised electrons. The positive metal ions are then attracted to the sea of delocalised electrons by strong electrostatic forces of attraction which are called metallic bonds.
Properties of Metals
1. Metals usually have high melting points. Metals have giant metallic lattice structure. Large amount of heat energy to break the strong metallic bonds and the lattice structure.
2. Metals are malleable (bent and pressed into shape) and ductile (drawn out into wires). The layers of atoms in pure metals can slide over each other without breaking the metallic bond.
3. Metals are good conductors of heat. When more heat is supplied, delocalised electrons gain kinetic energy which makes them diffuse faster into cooler regions of the metal. They quickly transfer the heat throughout the lattice structure. .
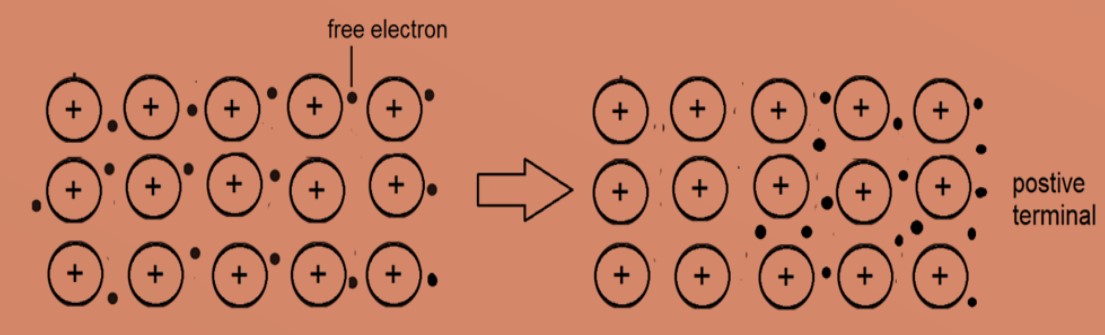
4. Metals are good conductors of electricity. The free electrons can move through the lattice towards positive terminal, when a voltage is applied across the metal.