Experimental Techniques (IGCSE)
2.2.1 Criteria of purity
Core
• Demonstrate knowledge and understanding of paper chromatography
This method can be used to separate a mixture of substances and identify them.
Steps
1. Prepare concentrated solution of the samples.
2. Draw a pencil line on a piece of chromatography paper.
3. Then, place a drop of each sample on the pencil line.
4. Next, place the paper into a beaker with a suitable solvent. Ensure the pencil line is above the level of solvent.
5. Cover the beaker with a lid.
6. Solvent travels up the paper, dissolves the sample and carrying the components of the sample along
7. Components that are more soluble in the solvent will travel further
8. When the solvent is near the top of the paper, remove the paper. Mark a line in pencil on it, to show where the solvent reached.
9. Put the paper in an oven to dry out.
10. A chromatogram is obtained.
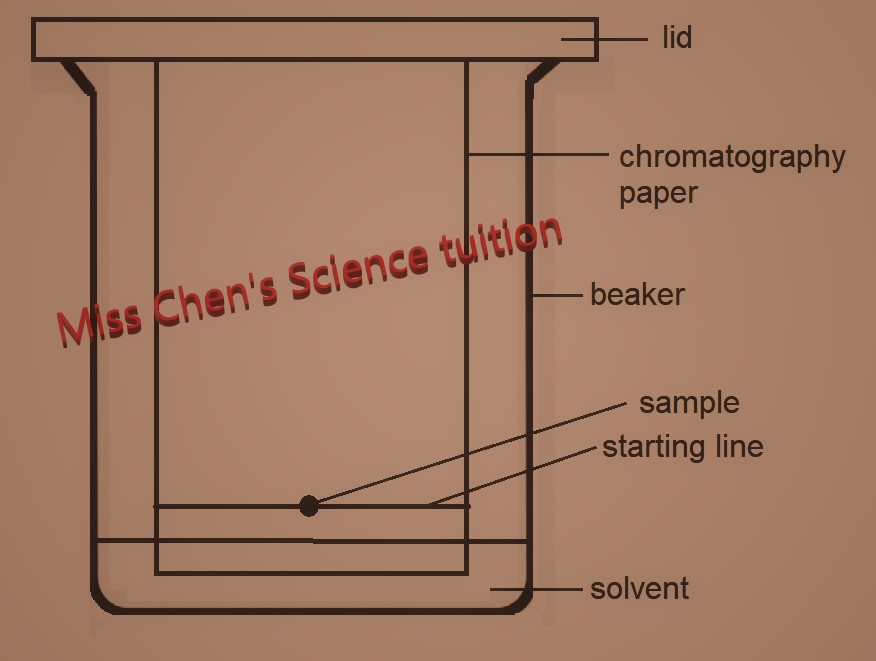
Uses- on a small scale to identify substances, check the purity of substances and help in crime detection.
• Interpret simple chromatograms.
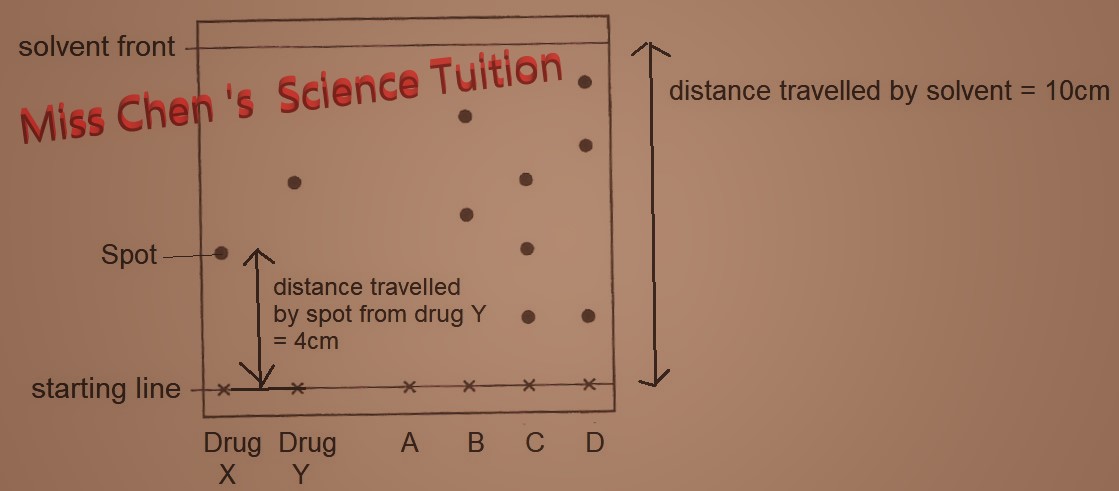
-B is a mixture of two substances as there are two spots on the chromatogram.
-Drug X and Y are pure substances as there is only one spot on the chromatogram.
-Sample A is insoluble in the solvent thus there is no spot seen.
-Sample C contain drug Y as C has a spot at same height as spot from drug Y.
Supplement
1. Interpret simple chromatograms, including the use of Rf values.
Rf values = distance from starting line to centre of spot
Distance from starting line to solvent front
Example from diagram: Rf value of spot from drug Y = 4/10 = 0.4
The more soluble the component in the solvent, the higher the Rf values.
You can look up list of Rf tables to identify the substances as the Rf value of a compound is always the same for a given solvent, under the same conditions.
2.Outline how chromatography techniques can be applied to colourless substances by exposing chromatograms to substances called locating agents. (Knowledge of specific locating agents is not required.)
If the spots are colourless on the chromatogram, spray the chromatogram with a locating agent. This will make the spots coloured and visible.
2.2.2 Methods of purification Core
• Describe and explain methods of purification by the use of a suitable solvent, filtration, crystallisation and distillation (including use of a fractionating column). (Refer to the fractional distillation of petroleum in section 14.2 and products of fermentation in section 14.6.)
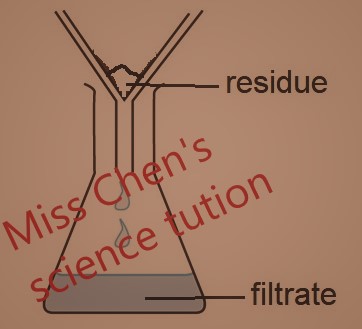
1. Filtration - Separate insoluble solid from solution or liquid.
For example, chalk is insoluble in water. The chalk is trapped in the filter paper, while the water passes through. The trapped solid is called the residue. The solution or liquid passes through is the filtrate. Water is the filtrate.
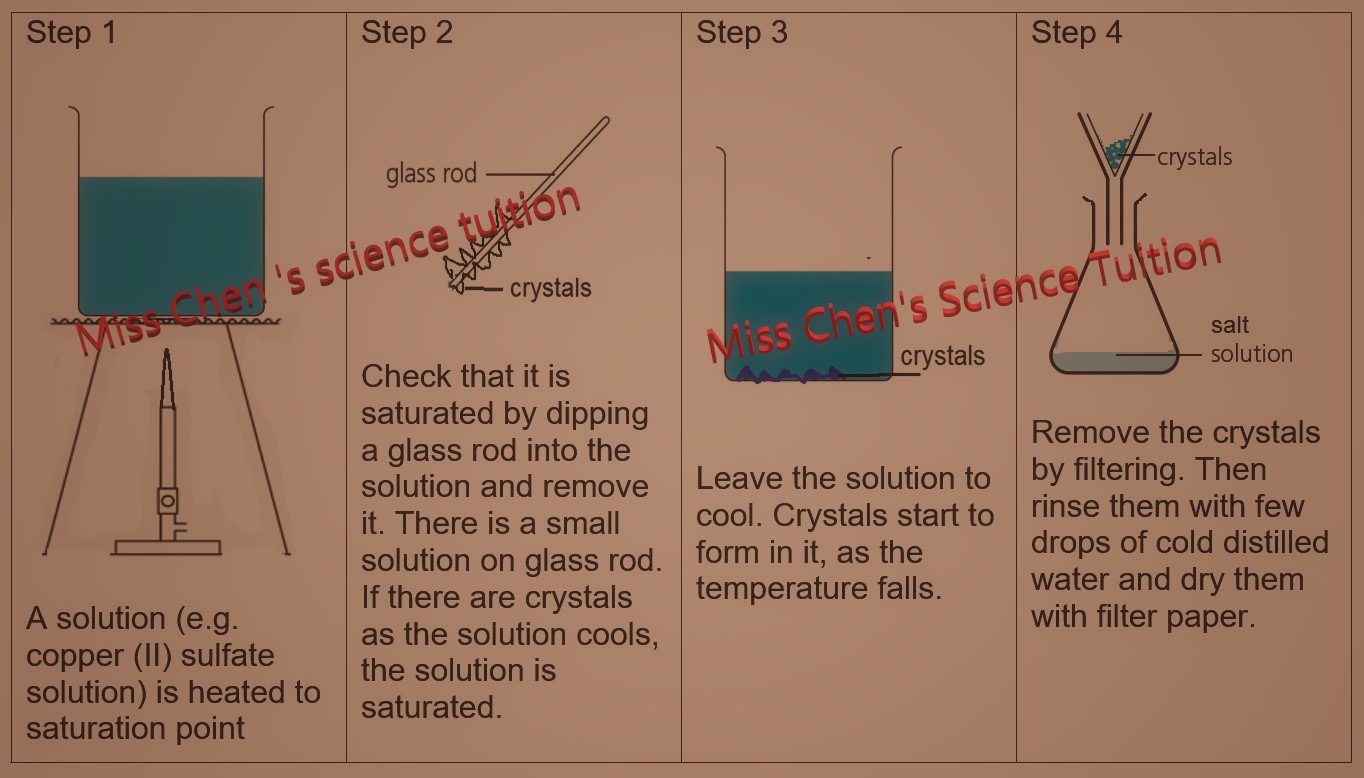
2. Crystallisation- to separate soluble solid from a solution.
3. Simple distillation- to obtain the solvent/liquid from a solution.
.jpg)
The set-up is shown above. It could be used to obtain water from salt water.
i. Heat the seawater in the flask. Water boils to become water vapour and enter the condenser, leaving salt behind.
ii.The hot vapour condenses to water in the cold condenser.
iii.The pure water flows into the beaker and is collected as distillate.
4. Fractional distillation
This is used to separate a mixture of miscible liquids from a solution. The liquids have different boiling points.
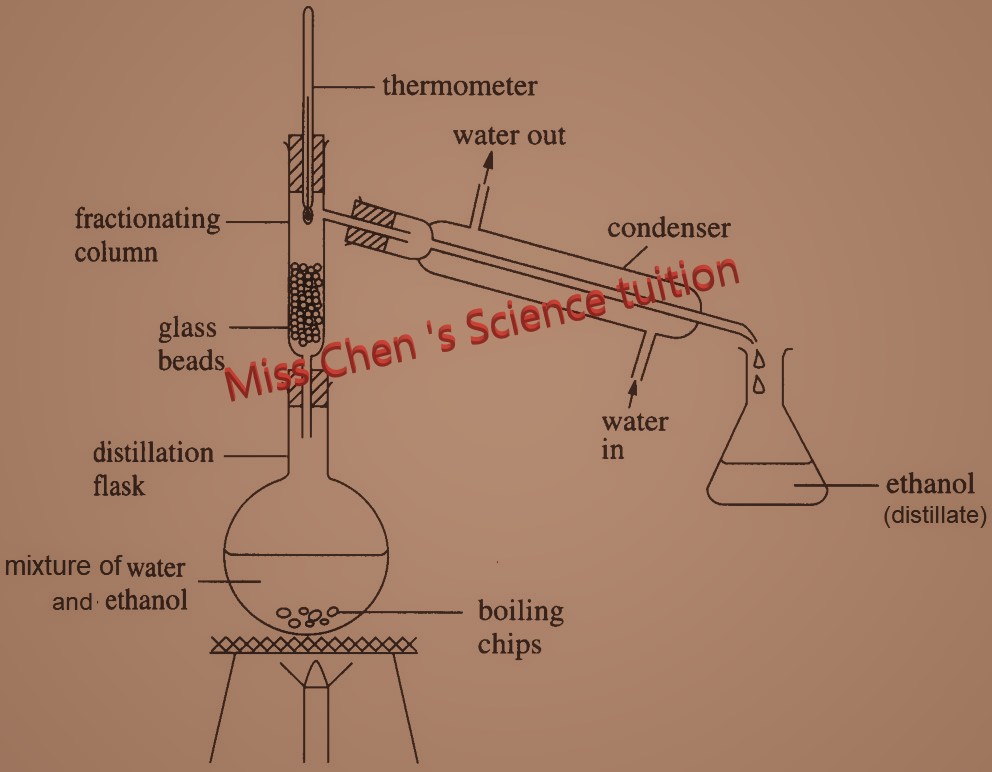
You can use it to separate a mixture of ethanol and water. The apparatus set up is shown above.
These are the steps:
i. Heat the mixture in the flask. Ethanol and water boils too. A mixture of ethanol and water vapours rises up the column.
ii. The vapours condense on the glass beads in the column, making them hot.
iii. When the beads reach about 78 °C. Only the water vapour condenses to become water. So water flows back into the flask. The ethanol vapour enters the condenser.
iv. Ethanol vapour condenses into pure liquid ethanol which flows into the beaker.
v. When the thermometer reading rises above 78 °C , is a sign that all the ethanol has gone.
vi. When temperature reaches 100Oc, liquid water will be distilled out.
Uses of Fractional distillation in industry
i. To refine crude oil into petrol, diesel and other useful fractions in petroleum industry.
ii. To obtain ethanol from fermentation, using glucose solution.
iii. To obtain oxygen, nitrogen and other gases from liquified air.
- Suggest suitable purification techniques, given information about the substances involved.
| Method of separation |
Used to separate |
Example |
| Filtration |
Insoluble solid from a solution or liquid |
Sand and water |
| Crystallisation |
Soluble solid from a solution |
Copper (II) sufate crystals from copper (II) sulfate solutions |
| Simple distillation |
Solvent from a solution |
Water from seawater |
| Fractional distillation |
Miscible liquids from a solution |
Ethanol and water |
| Paper chromatography |
Different substances from a solution |
Dyes in ink |