Energy Changes (IGCSE/ O Levels)
| Endothermic reactions take in energy from their surroundings. |
Exothermic reactions give out energy. |
| Photosynthesis, thermal decomposition, barium hydroxide reacts with ammonium chloride |
the neutralisation of acids by alkalis, the combustion of fuels, respiration in your body cells |
| Breaking bonds takes in energy. |
Making bonds releases energy. |
| If the energy taken in to break bonds is greater than the energy released in making bonds, the reaction is endothermic. ∆H is positive |
If the energy taken in to break bonds is less than the energy released in making bonds, the reaction is exothermic. ∆H is negative. |
Calculating the energy changes in reactions.
1. Write balanced equation.
2. Write out the structural formula showing the bonds in the equation.
3. Energy change = total energy taken in to break bonds in reactants - total energy taken given out to form bonds in reactants
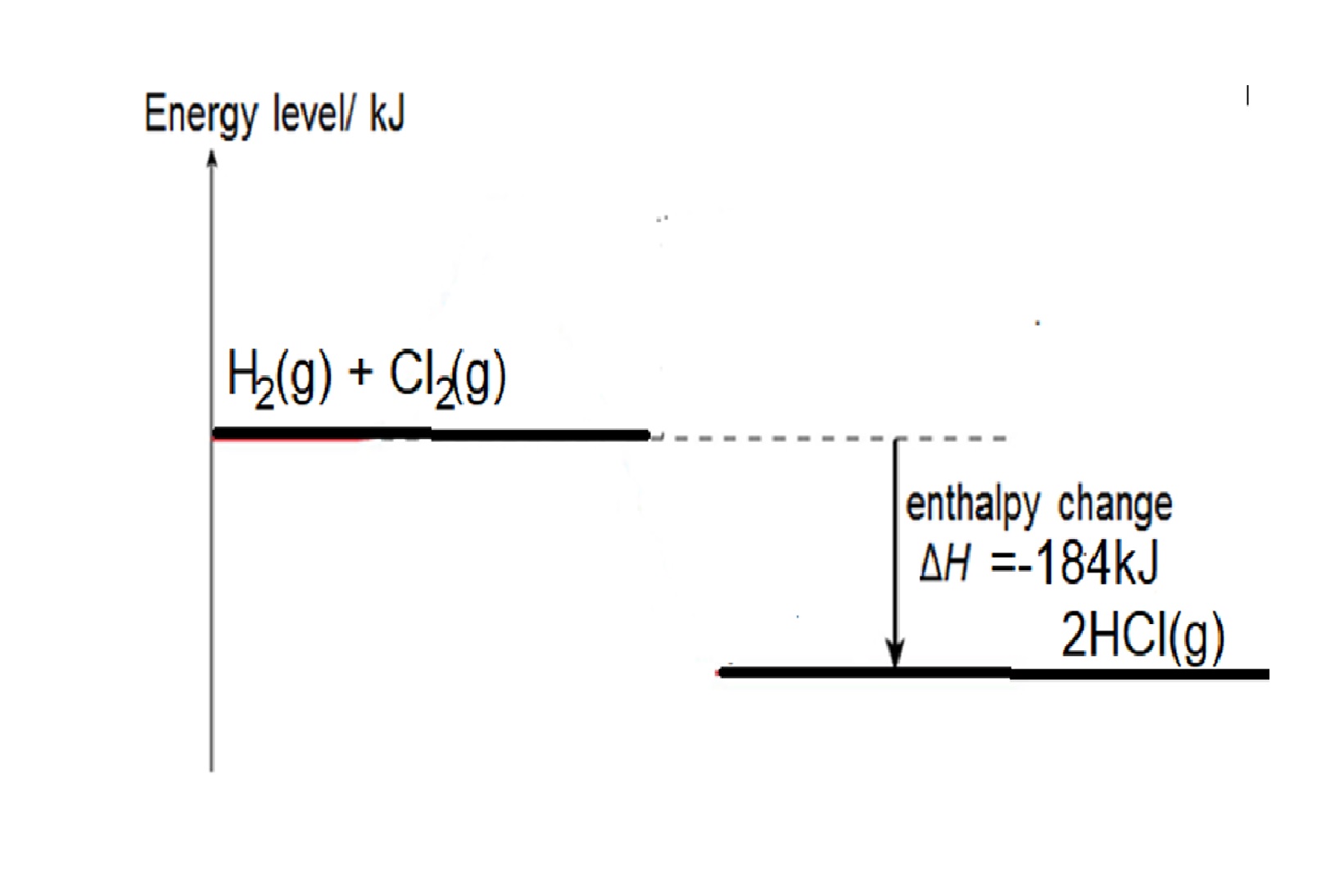
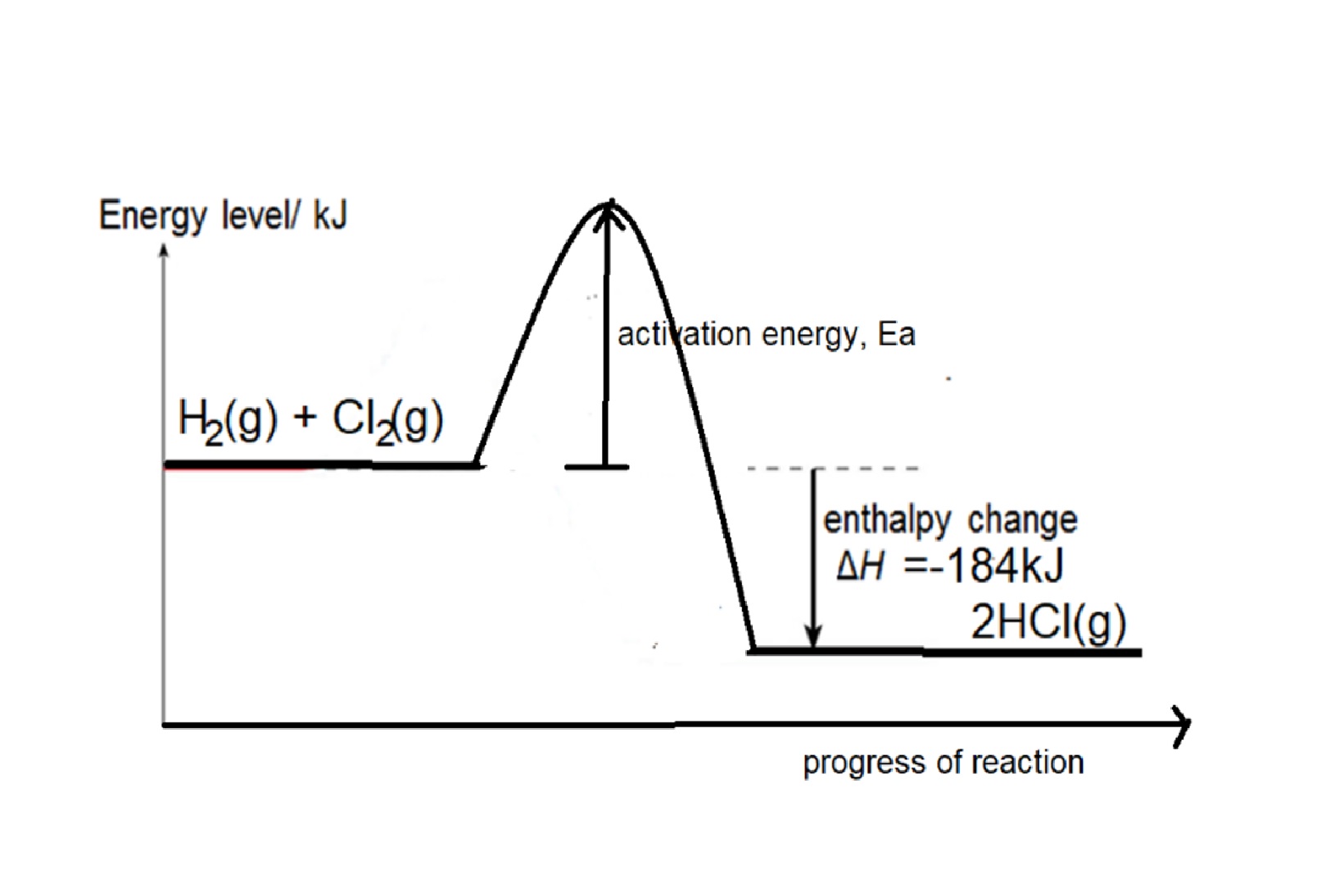
Eg1: Given bond energy of H-H is 436kJ, Cl-Cl bond is 242kJ and H-Cl is 431kJ, find the energy change when hydrogen reacts with chlorine to form hydrogen chloride,
Step 1: write balanced chemical equation: H2 + Cl2 → 2HCl
Step 2: structural formula showing the bonds in the equation: H-H + Cl-Cl → 2 H-Cl
Step 3: energy change = bond energy of (H-H + Cl-Cl) – 2 X bond energy of H-Cl
= 436 +242 - (2 x 431) = -184kJ
Reaction is exothermic as total energy taken in to break bonds in H2 and Cl2 is less than total energy released to form bonds in HCl.
Energy Level diagram
Energy Profile diagram
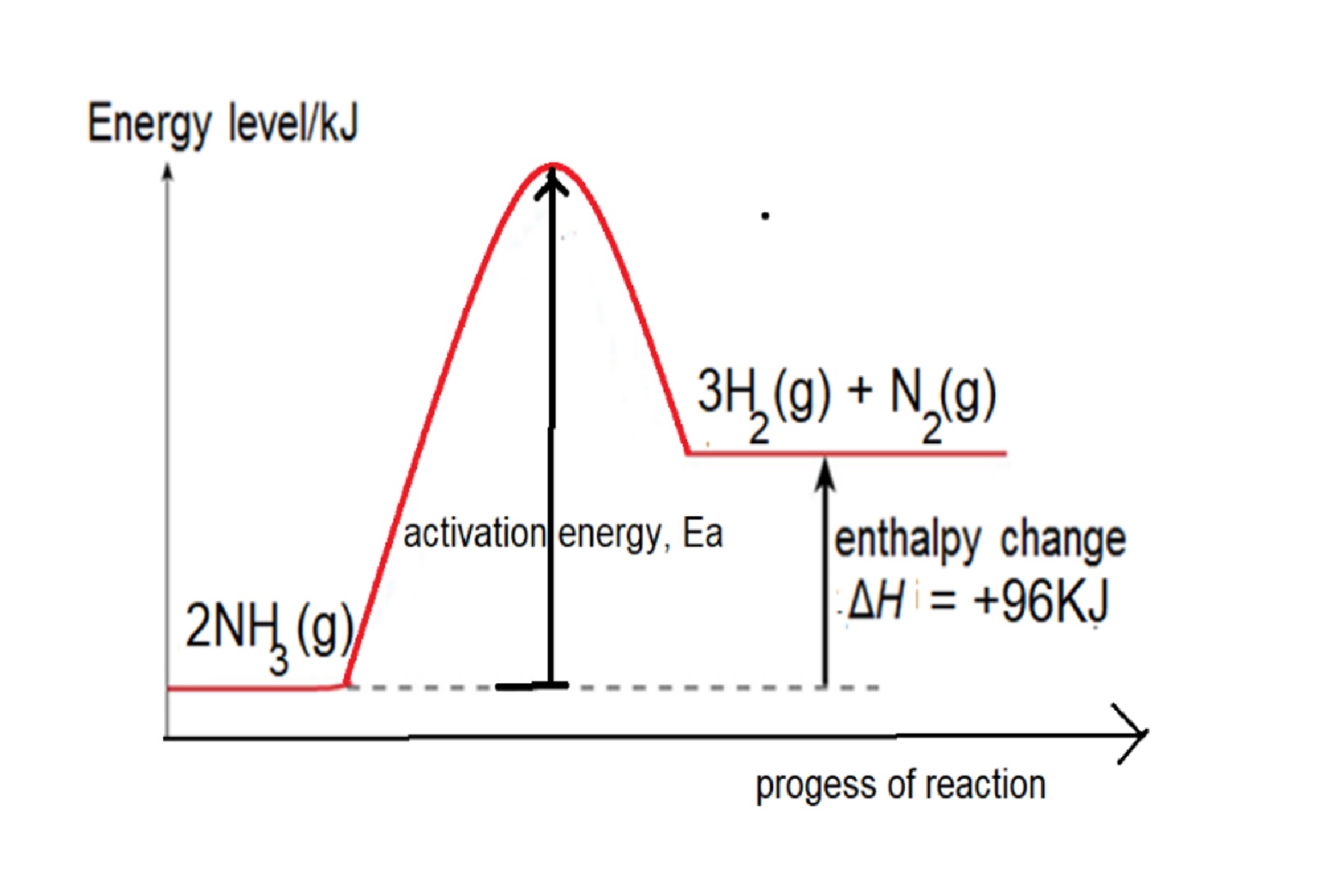
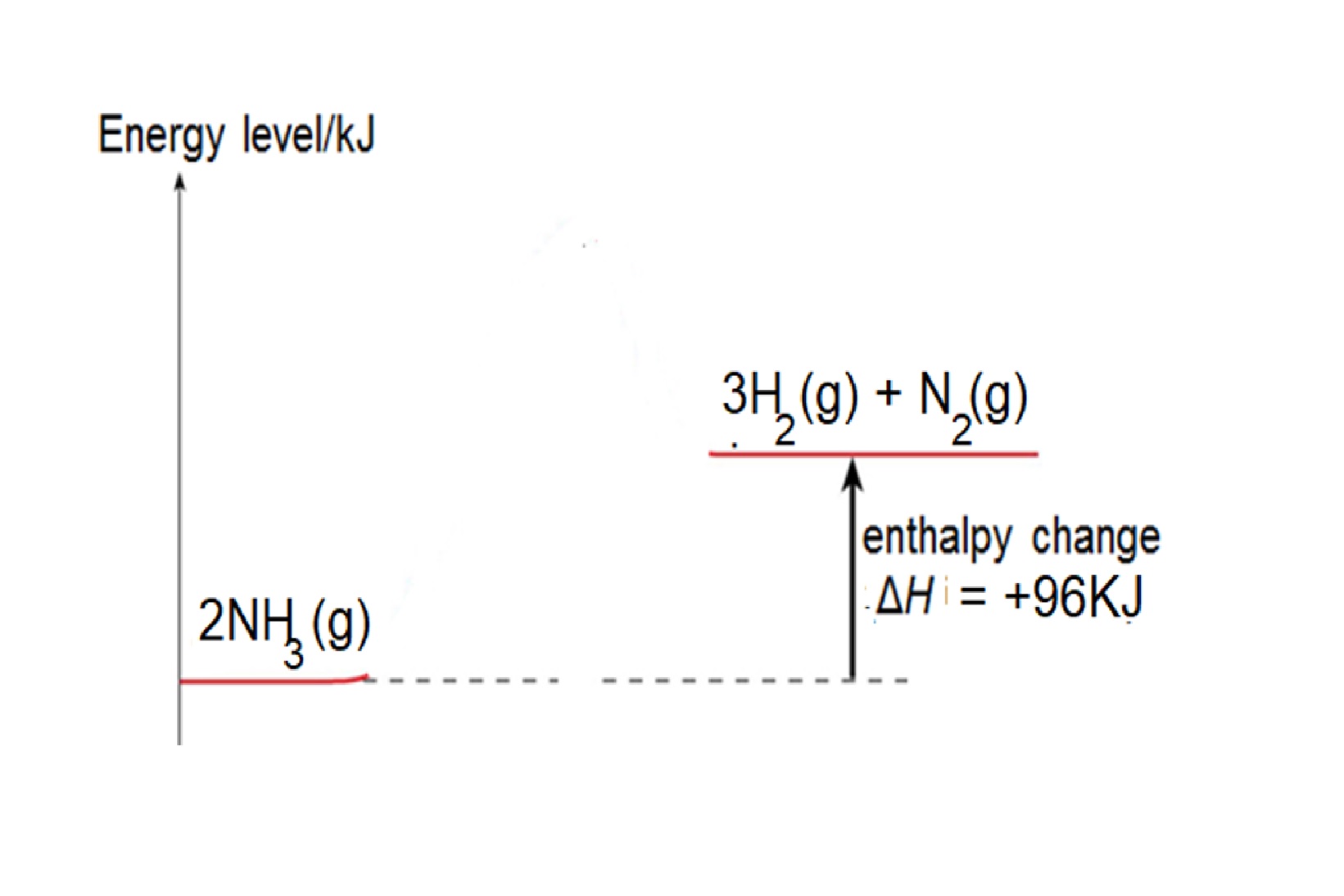
Eg2: Given bond energy of N-H is 391kJ, N≡N is 946kJ and H-H is 436kJ, find the energy change when ammonia decomposes to form nitrogen and hydrogen.
Step 1: write balanced chemical equation: 2NH3 → 3H2 + N2
H
|
Step 2: structural formula showing the bonds in the equation: 2 H-N-H → 3H-H + N≡N
Step 3: energy change = 6 X bond energy of N-H – [3 X bond energy of H-H + bond energy of N≡N] =(6 x 391) – [ (3x436) – 946] = +96kJ
Reaction is endothermic as total energy taken in to break bonds in NH3 is more than total energy released to form bonds in H2 and N2.
Energy Level diagram
Energy Profile Diagram